Storm Clouds Of Jupiter





Storm Clouds of Jupiter
More Posts from Sergioballester-blog and Others
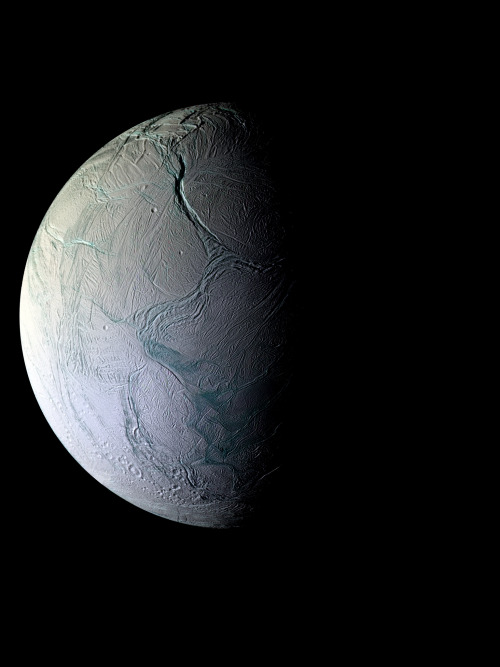
Image of the moon of Saturn, Enceladus, taken by the Cassini spacecraft
Image credit: NASA / JPL

The Pale Blue Dot
The “Pale Blue Dot” is a photograph of planet Earth taken in 1990 by Voyager 1 from a record distance, showing it against the vastness of space.
By request of Carl Sagan, NASA commanded the Voyager 1 spacecraft, having completed its primary mission and now leaving the Solar System, to turn its camera around and to take a photograph of Earth across a great expanse of space. Reflecting on this picture (now considered one of the most important pictures in all of human history) Carl Sagan said:
“From this distant vantage point, the Earth might not seem of particular interest. But for us, it’s different. Consider again that dot. That’s here, that’s home, that’s us. On it everyone you love, everyone you know, everyone you ever heard of, every human being who ever was, lived out their lives. The aggregate of our joy and suffering, thousands of confident religions, ideologies, and economic doctrines, every hunter and forager, every hero and coward, every creator and destroyer of civilization, every king and peasant, every young couple in love, every mother and father, hopeful child, inventor and explorer, every teacher of morals, every corrupt politician, every “superstar,” every “supreme leader,” every saint and sinner in the history of our species lived there – on a mote of dust suspended in a sunbeam.
The Earth is a very small stage in a vast cosmic arena. Think of the rivers of blood spilled by all those generals and emperors so that, in glory and triumph, they could become the momentary masters of a fraction of a dot. Think of the endless cruelties visited by the inhabitants of one corner of this pixel on the scarcely distinguishable inhabitants of some other corner, how frequent their misunderstandings, how eager they are to kill one another, how fervent their hatreds.
Our posturings, our imagined self-importance, the delusion that we have some privileged position in the Universe, are challenged by this point of pale light. Our planet is a lonely speck in the enveloping cosmic dark. In our obscurity, in all this vastness, there is no hint that help will come from elsewhere to save us from ourselves.
The Earth is the only world known so far to harbor life. There is nowhere else, at least in the near future, to which our species could migrate. Visit, yes. Settle, not yet. Like it or not, for the moment the Earth is where we make our stand.
It has been said that astronomy is a humbling and character-building experience. There is perhaps no better demonstration of the folly of human conceits than this distant image of our tiny world. To me, it underscores our responsibility to deal more kindly with one another, and to preserve and cherish the pale blue dot, the only home we’ve ever known.”
The first palaeontologist on Mars

(Image: Artist’s impression of NASA’s Perseverance rover on Mars)
Today NASA’s Perseverance rover landed on Mars. I don’t usually talk astronomy on this blog, but this time it’s relevant because—as you might have read—Perseverance is more or less the first palaeontologist on Mars!
Let me explain.

(Image: Satellite topography map of Jezero Crater, the site where Perseverance landed)
The site where Perseverance is landing, Jezero Crater, is a meteor impact crater near Mars’s Equator (say that 10 times fast!). It has evidence of a delta—the geomorphic feature that occurs when running water enters a large body of water. Orbital analyses also suggest it’s filled with carbonate rock—the kind that tend to deposit at the bottom of bodies of water.
Jezero Crater is not filled with water today. But the evidence strongly suggests it once was. If we’re going to find evidence of life on Mars, this is a good place to start looking.
Microbial fossils
When you think of fossils, most people think of giant T. rex skeletons, or frozen woolly mammoths, or neanderthal skulls. Maybe you’ve been around the block a bit, and you think about corals, or plant fossils, or tiny fossil shells. But some of the most common and important fossils on Earth are even tinier. Microbial fossils are commonly made by bacteria, archaea, and the like.

(Image: A cross-section of a stromatolite fossil, showing the multiple layers)
Some of the earliest fossils on earth are called stromatolites. They occur when bacterial colonies grow together in a mat—then, over time, sediment deposits over the colony, and the bacteria form another layer on top of the previous layer. Over time, many layers can be formed.

(Image: Helium Ion Microscopy image of iron oxide filaments formed by bacteria)
Although we breathe in oxygen and breathe out carbon dioxide, many microbes are not quite so restricted, and can breathe anything from sulphur to iron to methane or ammonia. When they do this, they often leave behind solid waste products, such as the above iron oxide filaments, that give away their presence. We can tell these apart from normal minerals in a number of ways, including by the relative proportions of different isotopes in them.

(Image: Schematic digram showing how molecular fossils form and are studied)
However, some of the most important fossils are molecular fossils. Living organisms produce a variety of different organic molecules; even long after the bodies of these organisms decay, those molecules can stay behind in an altered form for millions or even billions of years. If we’re looking for evidence of life on Mars, this might be our best bet.
Enter Perseverance

(Image: Diagram of Perseverance rover showing different instruments)
The Perseverance rover is overall similar in design to the Curiosity rover that landed in 2012, but there are some key differences—and most relevant here is that it’s a geological powerhouse. It’s got a number of instruments designed to carry out detailed geologic investigations:
RIMFAX is a ground-penetrating Radar unit. Like normal Radar, it works by sending radio waves into the ground; different materials affect the radio waves differently, as do transitions between different materials. This will allow us to, for the first time, study the geology of Mars below the surface to get an idea of what has been going on down there.

(Image: This is the kind of result produced by ground-penetrating radar—a rough image of the stratigraphy below the surface.)
PIXL (Planetary Instrument for X-ray Lithochemistry) shoots x-rays at samples and examines how they fluoresce in reaction. This allows for the detection of the elemental composition of a sample—helping us better understand the geology of the area, and potentially detect signatures of life.
SuperCam is a multi-function laser spectrometer that uses four different spectroscopy methods to examine the composition of samples. They all work in similar ways—essentially, different molecules react to laser stimulation differently, and different amounts of energy are required to make different molecules vibrate. The way that these molecules react can help us identify their composition, and the hope is that this may allow us to detect molecular fossils (these methods allow us to detect molecular fossils on Earth!)
SHERLOC (Scanning Habitable Environments with Raman & Luminescence for Organics & Chemicals) is another spectroscopic instrument—this one, however, is more precise, and optimised for detecting trace biosignatures in samples. It works similar to the above, using an ultraviolet laser to scan a 7 × 7 mm zone for evidence of organic compounds.
In addition to studying samples in situ, Perseverance will package small samples and leave them behind on Mars. A planned future mission will collect these packaged samples and launch them into space, where an orbiter will collect them and—hopefully—return them to Earth. This would be the first time that samples have ever been recovered from Mars, and would go a long way in increasing our understanding of the Martian environment and geology.
There’s no way of knowing yet what Perseverance will find—but even the fact that a robot palaeontologist is on Mars is incredibly exciting. Here’s to many years of discovery!
Stars Make Firework Supplies!
The next time you see fireworks, take a moment to celebrate the cosmic pyrotechnics that made them possible. From the oxygen and potassium that help fireworks burn to the aluminum that makes sparklers sparkle, most of the elements in the universe wouldn’t be here without stars.
From the time the universe was only a few minutes old until it was about 400 million years old, the cosmos was made of just hydrogen, helium and a teensy bit of lithium. It took some stellar activity to produce the rest of the elements!

Stars are element factories
Even after more than 13 billion years, the hydrogen and helium that formed soon after the big bang still make up over 90 percent of the atoms in the cosmos. Most of the other elements come from stars.

Stars began popping into the universe about 400 million years after the big bang. That sounds like a long time, but it’s only about 3% of the universe’s current age!
Our Nancy Grace Roman Space Telescope will study the universe’s early days to help us learn more about how we went from a hot, soupy sea of atoms to the bigger cosmic structures we see today. We know hydrogen and helium atoms gravitated together to form stars, where atoms could fuse together to make new elements, but we're not sure when it began happening. Roman will help us find out.

The central parts of atoms, called nuclei, are super antisocial – it takes a lot of heat and pressure to force them close together. Strong gravity in the fiery cores of the first stars provided just the right conditions for hydrogen and helium atoms to combine to form more elements and generate energy. The same process continues today in stars like our Sun and provides some special firework supplies.
Carbon makes fireworks explode, helps launch them into the sky, and is even an ingredient in the “black snakes” that seem to grow out of tiny pellets. Fireworks glow pink with help from the element lithium. Both of these elements are created by average, Sun-like stars as they cycle from normal stars to red giants to white dwarfs.
Eventually stars release their elements into the cosmos, where they can be recycled into later generations of stars and planets. Sometimes they encounter cosmic rays, which are nuclei that have been boosted to high speed by the most energetic events in the universe. When cosmic rays collide with atoms, the impact can break them apart, forming simpler elements. That’s how we get boron, which can make fireworks green, and beryllium, which can make them silver or white!

Since massive stars have even stronger gravity in their cores, they can fuse more elements – all the way up to iron. (The process stops there because instead of producing energy, fusing iron is so hard to do that it uses up energy.)
That means the sodium that makes fireworks yellow, the aluminum that produces silver sparks (like in sparklers), and even the oxygen that helps fireworks ignite were all first made in stars, too! A lot of these more complex elements that we take for granted are actually pretty rare throughout the cosmos, adding up to less than 10 percent of the atoms in the universe combined!
Fusion in stars only got us through iron on the periodic table, so where do the rest of our elements come from? It’s what happens next in massive stars that produces some of the even more exotic elements.

Dying stars make elements too!
Once a star many times the Sun’s mass burns through its fuel, gravity is no longer held in check, and its core collapses under its own weight. There, atoms are crushed extremely close together – and they don’t like that! Eventually it reaches a breaking point and the star explodes as a brilliant supernova. Talk about fireworks! These exploding stars make elements like copper, which makes fireworks blue, and zinc, which creates a smoky effect.
Something similar can happen when a white dwarf star – the small, dense core left behind after a Sun-like star runs out of fuel – steals material from a neighboring star. These white dwarfs can explode as supernovae too, spewing elements like the calcium that makes fireworks orange into the cosmos.

When stars collide
White dwarfs aren’t the only “dead” stars that can shower their surroundings with new elements. Stars that are too massive to leave behind white dwarfs but not massive enough to create black holes end up as neutron stars.
If two of these extremely dense stellar skeletons collide, they can produce all kinds of elements, including the barium that makes fireworks bright green and the antimony that creates a glitter effect. Reading this on a phone or computer? You can thank crashing dead stars for some of the metals that make up your device, too!

As for most of the remaining elements we know of, we've only seen them in labs on Earth so far.
Sounds like we’ve got it all figured out, right? But there are still lots of open questions. Our Roman Space Telescope will help us learn more about how elements were created and distributed throughout galaxies. That’s important because the right materials had to come together to form the air we breathe, our bodies, the planet we live on, and yes – even fireworks!
So when you’re watching fireworks, think about their cosmic origins!
Learn more about the Roman Space Telescope at: https://roman.gsfc.nasa.gov/
Make sure to follow us on Tumblr for your regular dose of space: http://nasa.tumblr.com

Space Shuttle launch. 🌎🚀



After completing its SSO-A mission of launching a record breaking 64 satellites at once, the B5 Falcon 9 booster landed for the third time breaking another record!

An animation of Jupiter and Io by Nevan




jupiter

If you don’t fight for all women, you fight for no women. ✊🏿✊🏾✊🏽✊🏼✊🏻 (📸: Kennedy Dickerson)

Montage of Neptune and Triton by NASA on The Commons
-
 sorceress-nadira reblogged this · 5 months ago
sorceress-nadira reblogged this · 5 months ago -
 sorceress-nadira liked this · 5 months ago
sorceress-nadira liked this · 5 months ago -
 szkot-pielgrzym reblogged this · 5 months ago
szkot-pielgrzym reblogged this · 5 months ago -
 bigdaddytbone liked this · 7 months ago
bigdaddytbone liked this · 7 months ago -
 postcardsfromliminalplaces reblogged this · 1 year ago
postcardsfromliminalplaces reblogged this · 1 year ago -
 itznymph liked this · 1 year ago
itznymph liked this · 1 year ago -
 ama-mori liked this · 1 year ago
ama-mori liked this · 1 year ago -
 lee-eyecat liked this · 1 year ago
lee-eyecat liked this · 1 year ago -
 ninaizantina liked this · 1 year ago
ninaizantina liked this · 1 year ago -
 trammelled-red reblogged this · 1 year ago
trammelled-red reblogged this · 1 year ago -
 todayintokyo liked this · 1 year ago
todayintokyo liked this · 1 year ago -
 starrynightcat reblogged this · 1 year ago
starrynightcat reblogged this · 1 year ago -
 helga1031 liked this · 1 year ago
helga1031 liked this · 1 year ago -
 oldstuffnewstuff reblogged this · 1 year ago
oldstuffnewstuff reblogged this · 1 year ago -
 oswur reblogged this · 1 year ago
oswur reblogged this · 1 year ago -
 modalitate reblogged this · 1 year ago
modalitate reblogged this · 1 year ago -
 noswhoreatu liked this · 1 year ago
noswhoreatu liked this · 1 year ago -
 doctorhelm liked this · 1 year ago
doctorhelm liked this · 1 year ago -
 stratabilly reblogged this · 1 year ago
stratabilly reblogged this · 1 year ago -
 fanofeverythinginthetv liked this · 1 year ago
fanofeverythinginthetv liked this · 1 year ago -
 avidbeader liked this · 1 year ago
avidbeader liked this · 1 year ago -
 queen-stevie reblogged this · 1 year ago
queen-stevie reblogged this · 1 year ago -
 queen-stevie liked this · 1 year ago
queen-stevie liked this · 1 year ago -
 mizjoely reblogged this · 1 year ago
mizjoely reblogged this · 1 year ago -
 mizjoely liked this · 1 year ago
mizjoely liked this · 1 year ago -
 gloria64 liked this · 1 year ago
gloria64 liked this · 1 year ago -
 iluvwind reblogged this · 1 year ago
iluvwind reblogged this · 1 year ago -
 iluvwind liked this · 1 year ago
iluvwind liked this · 1 year ago -
 weltonsbitch liked this · 1 year ago
weltonsbitch liked this · 1 year ago -
 cuddlyalphonse reblogged this · 1 year ago
cuddlyalphonse reblogged this · 1 year ago -
 cuddlyalphonse liked this · 1 year ago
cuddlyalphonse liked this · 1 year ago -
 meet-my-skeleton-pals liked this · 1 year ago
meet-my-skeleton-pals liked this · 1 year ago -
 therealbucky05 reblogged this · 1 year ago
therealbucky05 reblogged this · 1 year ago -
 therealbucky05 liked this · 1 year ago
therealbucky05 liked this · 1 year ago -
 summerwages liked this · 1 year ago
summerwages liked this · 1 year ago -
 vtatters reblogged this · 1 year ago
vtatters reblogged this · 1 year ago -
 vtatters liked this · 1 year ago
vtatters liked this · 1 year ago -
 msc7007 reblogged this · 1 year ago
msc7007 reblogged this · 1 year ago -
 scorpio-animus reblogged this · 1 year ago
scorpio-animus reblogged this · 1 year ago -
 philosophalycon reblogged this · 1 year ago
philosophalycon reblogged this · 1 year ago