Stephanie Kwolek Was Born #OTD In 1923. She’s Most Famous For Inventing The Bulletproof Polymer Kevlar:

Stephanie Kwolek was born #OTD in 1923. She’s most famous for inventing the bulletproof polymer Kevlar: wp.me/p4aPLT-3dv
More Posts from Contradictiontonature and Others

I gotta split! Image of the Week - June 22, 2015
CIL:41466 - http://www.cellimagelibrary.org/images/41466
Description: Confocal image of a mitotic spindle in a dividing cell. The spindle is shown in yellow and the surrounding actin cytoskeleton is in blue. Sixth Prize, 2007 Olympus BioScapes Digital Imaging Competition.
Authors: Patricia Wadsworth and the 2007 Olympus Bioscapes Digital Imaging Competition®.
Licensing: Attribution Non-Commercial No Derivatives: This image is licensed under a Creative Commons Attribution, Non-Commercial, No Derivatives License

Even after someone is declared dead, life continues in the body, suggests a surprising new study with important implications.
Gene expression — when information stored in DNA is converted into instructions for making proteinsor other molecules — actually increases in some cases after death, according to the new paper, which tracked postmortem activity and is published in the journal Open Biology.
“Not all cells are ‘dead’ when an organism dies,” senior author Peter Noble of the University of Washington and Alabama State University told Seeker. “Different cell types have different life spans, generation times and resilience to extreme stress.”
In fact, some cells seem to fight to live after the organism has died.
“It is likely that some cells remain alive and are attempting to repair themselves, specifically stem cells,” Noble said.
Complement Pathways

Components of complement pathways of the immune system.
Classical Pathway: binds to the pathogen surface
C1 binds to phosphocholine on bacteria, which activates C1r to cleave C1s.
Activated C1s cleaves C4 to C4a and C4b.
C4b binds to the microbial surface and also binds C2.
C2 is cleaved to C2a and C2b by C1s, forming the C4bC2a complex.
The C4bC2a complex cleaves C3 to C3a and C3b.
C3b binds to the surface and causes opsonization.
MB-Lectin Pathway: uses mannin-binding lectin to bind to mannose-containing carbohydrates on the pathogen surface
Mannin-binding lectin (MBL) binds to the pathogen surface and activates MASP-2.
MASP-2 cleaves C4 to C4a and C4b.
C4b binds to the microbial surface and also binds C2.
C2 is cleaved to C2a and C2b by MASP-2, forming the C4bC2a complex.
The C4bC2a complex cleaves C3 to C3a and C3b.
C3b binds to the surface and causes opsonization.
Alternative Pathway: binds to the pathogen surface with spontaneously activated complement, amplifies C3b
C3b deposited by the C3 convertase binds to factor B.
Factor B is cleaved by factor D into Ba and Bb, forming the C3bBb complex.
The C3bBb complex cleaves C3 into C3a and C3b.
C3 spontaneously hydrolyzes to C3(H2O).
C3(H2O) binds to factor B, and factor D cleaves factor B.
Upon factor B cleavage, the C3(H2O)Bb complex is formed.
The C3(H2O)Bb complex cleaves C3 into C3a and C3b.
Factor B binds to C3b on the surface and is cleaved to Bb.
The race to map the human body — one cell at a time
The first time molecular biologist Greg Hannon flew through a tumour, he was astonished — and inspired. Using a virtual-reality model, Hannon and his colleagues at the University of Cambridge, UK, flew in and out of blood vessels, took stock of infiltrating immune cells and hatched an idea for an unprecedented tumour atlas.
“Holy crap!” he recalls thinking. “This is going to be just amazing.”
On 10 February, the London-based charity Cancer Research UK announced that Hannon’s team of molecular biologists, astronomers and game designers would receive up to £20 million (US$25 million) over the next five years to develop its interactive virtual-reality map of breast cancers. The tumour that Hannon flew through was a mock-up, but the real models will include data on the expression of thousands of genes and dozens of proteins in each cell of a tumour. The hope is that this spatial and functional detail could reveal more about the factors that influence a tumour’s response to treatment.
The project is just one of a string that aims to build a new generation of cell atlases: maps of organs or tumours that describe location and make-up of each cell in painstaking detail.

Marrow Christmas and a Happy New Smear!
A very seasonal smear made from red marrow extracted from the iliac crest of a donor’s pelvis prior to transplantation.
Happy Holidays everyone
i♡histo
The image amazingly captures a single moment in time during the development of thousands of red and white blood cells.
Many of the small cells that are visible, like the ones forming the snowman’s carrot nose, do not have a nucleus. These are brand new erythrocytes (red blood cells) that are ready to exit the bone and enter the blood stream.
The other, slightly larger cells that have nuclei, like the snowman’s eyes and his top button, are either precursors to these erythrocytes (they will mature and lose their nucleus) or are precursors to the other blood cells in our body, the leukocytes (white blood cells): lymphocytes, monocytes, neutrophils, eosinophils and basophils.
In addition, the bone marrow is home to the cells that form platelets. These are huge multinucleated cells aptly named megakaryocytes - perhaps the cell at the bottom right.
It is possible to identify each mature cell and its precursor based upon its morphology and staining at higher magnification. High or low levels of these cells can indicate disease or cancers of the blood.

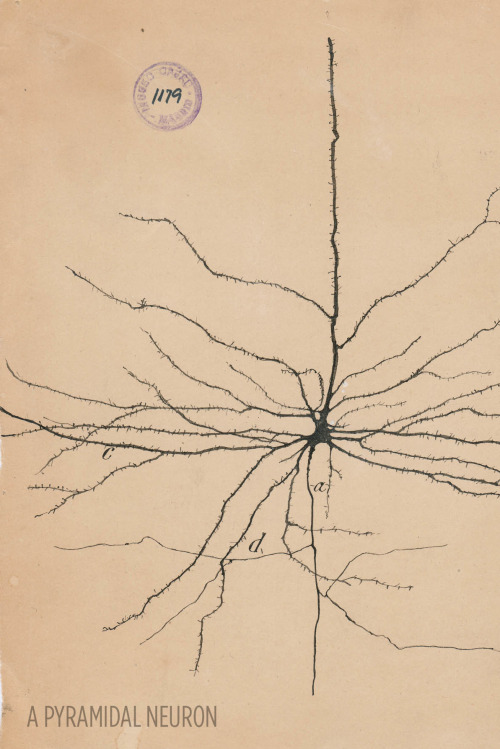



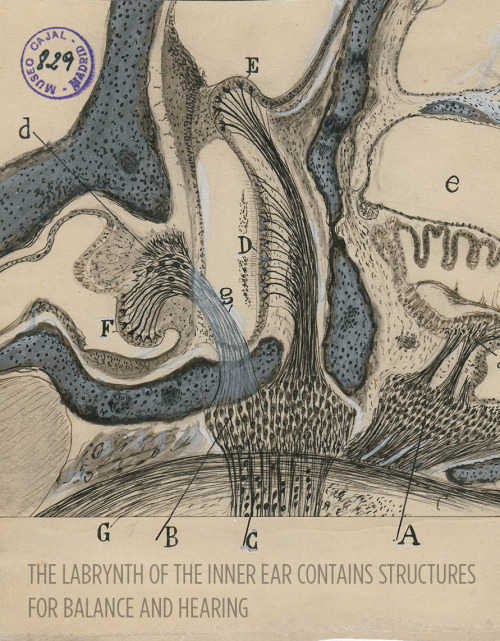


Art and science in beautiful conversation!
Here’s a 30-something Santiago Ramón y Cajal hanging out in his library:

For more, check out this article or visit the Weisman Art Museum in Minnesota before May 21.
Images courtesy of Instituto Cajal del Consjo Superior de Investigaciones Científicas, Madrid

A mighty membrane that twists and turns through the gut is starting the new year with a new classification: the structure, called the mesentery, has been upgraded to an organ.
Scientists have known about the structure, which connects a person’s small and large intestines to the abdominal wall and anchors them in place, according to the Mayo Clinic. However, until now, it was thought of as a number of distinct membranes by most scientists. Interestingly, in one of its earliest descriptions, none other than Leonardo da Vinci identified the membranes as a single structure, according to a recent review.










a mushroom rainbow to put the fun in fungi. cause they don’t need psilocybin to be magic. and though some mushrooms are coloured as a toxicity warning to predators, many others are brightly coloured to instead attract potential spore dispersers. see this for more on the bioluminescent mushrooms seen here. (photos)

Scientists have developed a new drug that could be a safer alternative to morphine for medical use. The researchers found that engineered variants of endomorphin, a naturally occurring chemical in the body, are as strong as morphine when it comes to killing pain.
On top of that, the medication doesn’t produce any of the unwanted side effects that come with opium-based drugs – such as being extremely addictive. At this point, the findings only relate to tests in rats, but it’s a promising start to what could be a powerful and less problematic painkiller.
Opioid pain medications are commonly used to treat severe and chronic pain, but in addition to their habit-forming qualities, patients also build up a tolerance to them over time. Hand in hand with their addictiveness, this can makes higher doses – and overdoses in drug abuse situations – dangerous. Overdoses can cause motor impairment and potentially fatal respiratory depression, resulting in thousands of deaths in the US every year.
-
 veek-chem liked this · 6 years ago
veek-chem liked this · 6 years ago -
 fractal-fluctuation liked this · 6 years ago
fractal-fluctuation liked this · 6 years ago -
 panda-poes reblogged this · 6 years ago
panda-poes reblogged this · 6 years ago -
 nichtsichtbar94 reblogged this · 7 years ago
nichtsichtbar94 reblogged this · 7 years ago -
 inthebloodstream-blog reblogged this · 7 years ago
inthebloodstream-blog reblogged this · 7 years ago -
 intermolecularity liked this · 7 years ago
intermolecularity liked this · 7 years ago -
 karolehernandez liked this · 7 years ago
karolehernandez liked this · 7 years ago -
 angaturama liked this · 7 years ago
angaturama liked this · 7 years ago -
 katyest liked this · 7 years ago
katyest liked this · 7 years ago -
 mad-chemist94 liked this · 7 years ago
mad-chemist94 liked this · 7 years ago -
 chot-dusit liked this · 7 years ago
chot-dusit liked this · 7 years ago -
 mekvall liked this · 7 years ago
mekvall liked this · 7 years ago -
 avogadromate-blog liked this · 7 years ago
avogadromate-blog liked this · 7 years ago -
 sorryimstudyin-blog1 liked this · 7 years ago
sorryimstudyin-blog1 liked this · 7 years ago -
 floatingchaote liked this · 7 years ago
floatingchaote liked this · 7 years ago -
 joshua-ghost-92 liked this · 7 years ago
joshua-ghost-92 liked this · 7 years ago -
 89thkitkat liked this · 7 years ago
89thkitkat liked this · 7 years ago -
 lovelymymind liked this · 7 years ago
lovelymymind liked this · 7 years ago -
 end-of-the-rabbithole reblogged this · 7 years ago
end-of-the-rabbithole reblogged this · 7 years ago -
 end-of-the-rabbithole liked this · 7 years ago
end-of-the-rabbithole liked this · 7 years ago -
 takipci1391 liked this · 7 years ago
takipci1391 liked this · 7 years ago -
 cat-lili liked this · 7 years ago
cat-lili liked this · 7 years ago -
 quantumfuckery liked this · 7 years ago
quantumfuckery liked this · 7 years ago -
 toonvailo reblogged this · 7 years ago
toonvailo reblogged this · 7 years ago -
 azulextraterestre liked this · 7 years ago
azulextraterestre liked this · 7 years ago -
 clareithromycin reblogged this · 7 years ago
clareithromycin reblogged this · 7 years ago -
 master7mindd reblogged this · 7 years ago
master7mindd reblogged this · 7 years ago -
 radix65 liked this · 7 years ago
radix65 liked this · 7 years ago -
 mommibot-blog-blog liked this · 7 years ago
mommibot-blog-blog liked this · 7 years ago -
 east-in-the-evening reblogged this · 7 years ago
east-in-the-evening reblogged this · 7 years ago -
 east-in-the-evening reblogged this · 7 years ago
east-in-the-evening reblogged this · 7 years ago -
 east-in-the-evening liked this · 7 years ago
east-in-the-evening liked this · 7 years ago -
 nevergonnaremember-blog liked this · 7 years ago
nevergonnaremember-blog liked this · 7 years ago -
 conderon-blog liked this · 7 years ago
conderon-blog liked this · 7 years ago -
 biomed017 liked this · 7 years ago
biomed017 liked this · 7 years ago -
 medical-sho liked this · 7 years ago
medical-sho liked this · 7 years ago -
 giorgioluciano reblogged this · 7 years ago
giorgioluciano reblogged this · 7 years ago
A pharmacist and a little science sideblog. "Knowledge belongs to humanity, and is the torch which illuminates the world." - Louis Pasteur
215 posts




